After a tenative start settling into Roma, my first full week in the Pier Luigi Luisi Synthetic Biology lab was no slow introduction; I found myself immediately involved in our first experiment to insert a DNA fragment into a plasmid. The first step in synthesising a protein Cristiano (postdoctoral fellow, and collaborating/advising scientist on this project) had inserted the fragment the week earlier. When I arrived we began a process called PCR (polymerase chain reaction) which is generally used for gene cloning and DNA analysis, and which we used to replicate the DNA that was inserted into the plasmid to test whether it had actually been inserted.
Without recounting the process in any technical detail at this point, needless to say this was a steep learning curve. Cristiano took me through the technique, which involves many steps with precise measurments, over four-days. Under his instruction, I also executed a number of simple steps so I could slowly begin to develop the technical skill necessary to eventually do the process myself. While it could be performed by a technician, to understand the process in terms of its biological affects at the level of the DNA requires having to fast track genetics and protein synthesis 101. Inserting a DNA fragment into a plasmid, however, doesn’t necessarily take the time we spent on it. Not primarily because Cristiano was teaching me and this slowed the process down -though it undoubtedly did -but because we didn’t ultimately get a successful result.
After running our first check to see if the DNA fragment had been replicated -and so had been cloned into the plasmid -we intially got a positive test. Using a standard electrophloresis gel test, the DNA fragment appeared at the point relative to the marker we used that it should, indicating it had been inserted.
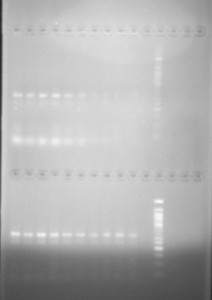
In this test the inserted DNA appears -the first white line reading top to bottom -at the expected point relative to the ladder which appears vertically on the right. The second white line is the remaining oligonucleotides we used to anneal the DNA molcules.
To double-check our initial positive result, before we sent it to another lab to read the DNA sequence, and so definately confirm that it in the plasmid, we ran a second test. After incubating the cloned bacteria with nutrient solution to gain a greater amount of bacterial cells with the insert and then isolating the plasmid DNA from the bacterial cultures, using a number of techniques (which are not really necessary to detail at this point) we performed a second eletrophloresis gel test. However, this time the test was negative. In contrast to the first text, the cloned plasmid with the insert did not appear.
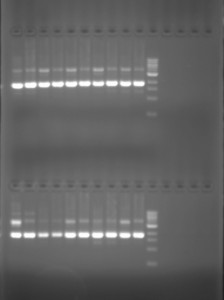
In our second test the inserted DNA does not appear. For the test to appear positive either one or three bands of DNA would have had to be present. One band would indicate a successful insertion, and three both a successful insertion and the presence of plasmid DNA without the insertion in circular and supercoiled form.
Given that we now had two contradictory results, one positive and one negative, we ran a third type of test -a double standard measure -which we once again examined using eletrophloresis. And once again, ufortunately the results were negative.
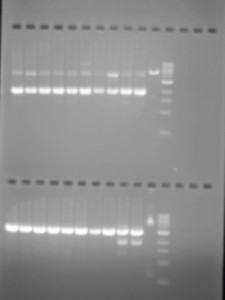
For this double standard test, we used two enzymes NcoI and NdeI to check whether the DNA was inserted into the plasmid. The NcoI test appears above and the NdeI test below. For the NcoI test either one or three white lines should appear: the cut plasmid with the DNA inserted, and two possible variations. For the NdeI test, two lines should appear: one for the cut plasmid and one for the uncut plasmid.
Having now confirmed the DNA fragment was not inserted into the plasmid, time was needed to consider why the insertion did not work. For me, this was an opportunity to digest the experimental week that was, both in its technical detail, broader framework of synthesising a protein, and in relation to my project goals.

